
产品展示
您当前的位置:首页 » 产品展示 » 安捷伦填充柱 » PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定

| 产品名称: | PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定 |
| 产品型号: | PQ/Porapak Q填充柱 |
| 品牌: | 1941 |
| 产品数量: | |
| 产品单价: | 面议 |
| 日期: | 2022-05-03 |
PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定的详细资料
医疗保健产品灭菌环氧乙烷残留测定医疗保健产品灭菌环氧乙烷残留测定 详细信息:
名称:填充柱
固定相:高分子小球
粒度:60-80目
规格:1-2m*内径2-3mm
型号:Porapak Qs
应用:
GB 18281.2-2000 医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
GB 18281.2-2015医疗保健产品灭菌 环氧乙烷 第1部分:医疗器械灭菌过程的开发、确认和常规控制的要求
GB 18281.2-2015医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
GB 18281.2-2015医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
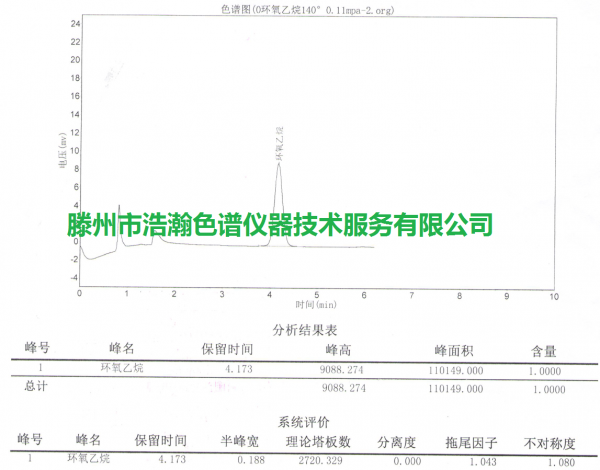
浩瀚色谱(山东)应用技术开发有限公司建立气相色谱法检测口罩、防护服等医疗防护用品中环氧乙烷的残留量,为生产厂家改进灭菌工艺提供数据支持。气相色谱法采用顶空进样、FID检测器,环氧乙烷的质量浓度在0.5~8 μg/mL范围内与色谱峰面积线性关系良好,检出限为0.10 μg/g,定量限为0.35 μg/g。环氧乙烷测定结果的相对标准偏差为1.73%(n=6),平均加标回收率为94.3%~99.3%。该方法操作简便,精密度、准确度较高,适用于医疗防护用品中环氧乙烷的测定。
医疗保健产品灭菌环氧乙烷残留测定 测试谱图:



Determination of Sterilized Ethylene Oxide Residues in Healthcare Products
Sterilization Ethylene Oxide Residue Determination of Healthcare Products Details:
Name: Packed Column
Statio
Granularity: 60-80 mesh
Specifications: 1-2m* inner diameter 2-3mm
Model: Porapak Qs
application:
GB 18281.2-2000 Sterilization of health care products - Biological indicators - Part 2: Biological indicators for sterilization of ethylene oxide
GB 18281.2-2015 Sterilization of Healthcare Products - Ethylene Oxide - Part 1: Requirements for the Development, Validation and Routine Co
GB 18281.2-2015 Sterilization of Health Care Products Biological Indicators Part 2: Biological Indicators for Ethylene Oxide Sterilization
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has established a gas chromatography method to detect the residual amount of ethylene oxide in medical protective equipment such as masks and protective clothing, and provide data support for manufacturers to improve the sterilization process. Gas chromatography adopts headspace injection and FID detector. The mass co
Determination of Sterilized Ethylene Oxide Residues in Healthcare Products Test Spectrum: