
您当前的位置:首页 » 产品展示 » 填充柱 » PEG-20M 药物中残留环氧丙烷的检测毛细管柱

| 产品名称: | PEG-20M 药物中残留环氧丙烷的检测毛细管柱 |
| 产品型号: | PEG-20M |
| 品牌: | 1941 |
| 产品数量: | |
| 产品单价: | 面议 |
| 日期: | 2022-04-27 |
PEG-20M 药物中残留环氧丙烷的检测毛细管柱的详细资料
药物中残留环氧丙烷的检测毛细管柱药物中残留环氧丙烷的检测毛细管柱 详细信息:
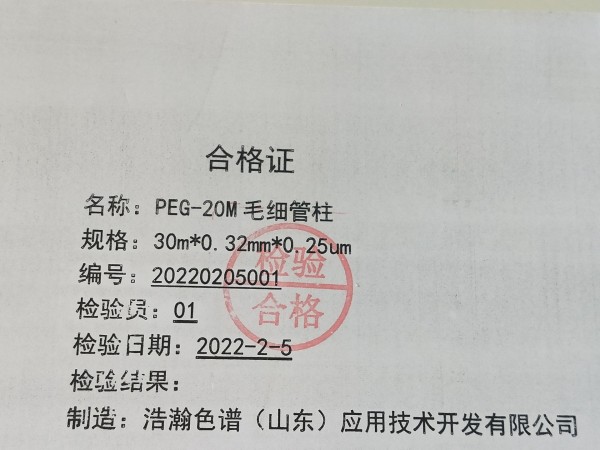
名称:毛细管柱
固定相:聚乙二醇
规格:30m*0.25mm*0.25um
型号:HH-WAX
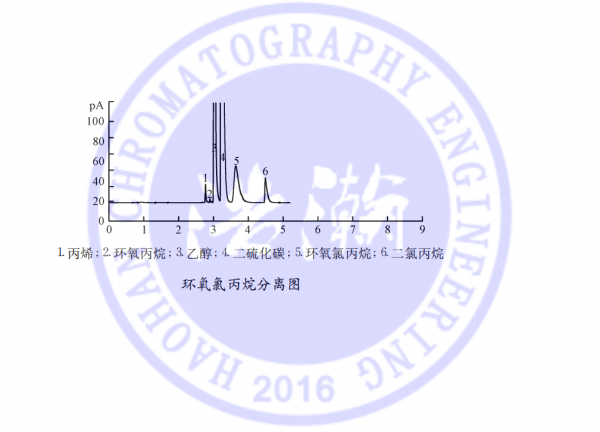
应用:顶空气相色谱法测定药品中环氧氯丙烷溶剂残留
目前,在国内以左奥硝唑为活性成分上市销售的制剂左奥硝唑氯化钠注射液,该药属硝基咪唑衍生物类抗厌氧菌药,为奥硝唑的左旋体。临床试验结果表明,左奥硝唑氯化钠注射液与奥硝唑氯化钠注射液相比,在减少头晕、嗜睡不良反应发生率方面,具有临床统计学意义。
环氧丙烷为合成左奥硝唑的起始原料s-环氧氯丙烷中的工艺杂质,环氧丙烷为国际癌症研究所(iarc)列为人类可疑致癌化学物,其具有致基因突变的警示结构,ichm7分类为1类基因毒性杂质,因此,能够检测左奥硝唑氯化钠注射液中活性成分中残留环氧丙烷的方法对于控制临床用药的安全性具有非常重要的意义。
浩瀚色谱(山东)应用技术开发有限公司研发一种药物中残留环氧丙烷的检测方法,所述的检测方法采用顶空气相色谱法,药物的稀释剂为N,N‑二甲基甲酰胺。采用该检测方法可以有效地检测药物例如奥硝唑、或左奥硝唑中可能存在环氧丙烷的残留情况。
药物中残留环氧丙烷的检测毛细管柱 测试谱图:


.jpg)
.jpg)








Capillary Column for Detection of Residual Propylene Oxide in Drugs
Capillary column for the detection of residual propylene oxide in pharmaceuticals Details:
Name: capillary column
Statio
Specifications: 30m*0.25mm*0.25um
Model: HH-WAX
Application: Determination of Epichlorohydrin Solvent Residues in Drugs by Headspace Gas Chromatography
At present, o
Propylene oxide is a process impurity in s-epichlorohydrin, the starting material for synthesizing levornidazole. Propylene oxide is listed as a suspected carcinogen by the Internatio
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has developed a detection method for residual propylene oxide in drugs. The detection method adopts headspace gas chromatography, and the diluent of the drug is N,N-dimethylformamide. The detection method can effectively detect the possible residual situation of propylene oxide in drugs such as ornidazole or levoornidazole.
Capillary column for detection of residual propylene oxide in pharmaceuticals Test chromatogram: